Selinexor and Backbone Treatments of Multiple Myeloma Patients
Summary
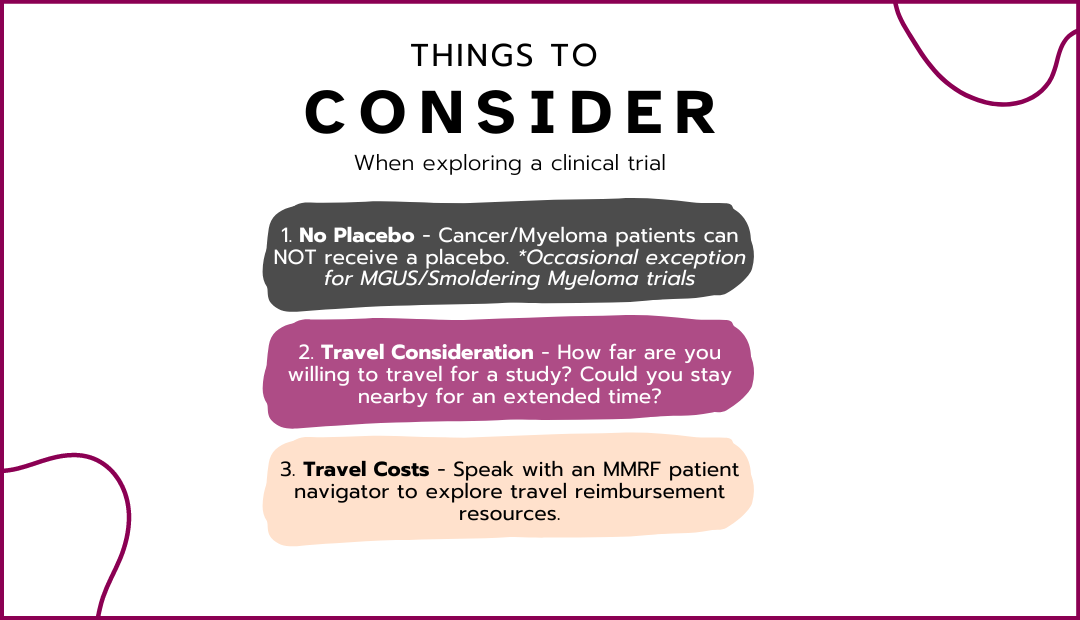
Trial Summary
This study will independently assess the efficacy and safety of 11 combination therapies in 12 arms, in dose-escalation/-evaluation and expansion phases, for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) and newly diagnosed multiple myeloma (NDMM). The combinations to be evaluated are: * Arm 1: Selinexor + dexamethasone + pomalidomide (SPd); enrollment complete * Arm 2: Selinexor + dexamethasone + bortezomib (SVd); enrollment complete * Arm 3: Selinexor + dexamethasone + lenalidomide (SRd) in RRMM; enrollment complete * Arm 4: Selinexor + dexamethasone + pomalidomide + bortezomib (SPVd); enrollment complete * Arm 5: Selinexor + dexamethasone + daratumumab (SDd); enrollment complete * Arm 6: Selinexor + dexamethasone + carfilzomib (SKd); enrollment complete * Arm 7: Selinexor + dexamethasone + lenalidomide (SRd) in NDMM; enrollment complete * Arm 8: Selinexor + dexamethasone + ixazomib (SNd); enrollment complete * Arm 9: Selinexor + dexamethasone + pomalidomide + elotuzumab (SPEd); enrollment complete * Arm 10: Selinexor + dexamethasone + belantamab mafodotin (SBd); enrollment complete * Arm 11: Selinexor + dexamethasone + pomalidomide + daratumumab (SDPd); enrollment complete * Arm 12: Selinexor + dexamethasone + mezigdomide (SMd); actively recruiting Selinexor pharmacokinetics: * PK Run-in (Days 1-14): Starting in protocol version 8.0, patients enrolled to any arm in the Dose Escalation Phase (i.e., Arm 4 \[SPVd\], Arm 6 \[SKd\], Arm 8 \[SNd\], Arm 9 \[SPEd\], Arm 10 \[SBd\], and Arm 11 \[SDPd\]) will also first be enrolled to a pharmacokinetics (PK) Run-in period until 9 patients have been enrolled to this period to evaluate the PK of selinexor before and after co-administration with a strong CYP3A4 inhibitor. This run-in period does not apply to Arm 12 (SMd).
This study will independently assess the efficacy and safety of 11 combination therapies in 12 arms, in dose-escalation/-evaluation and expansion phases, for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) and newly diagnosed multiple myeloma (NDMM). The combinations to be evaluated are: * Arm 1: Selinexor + dexamethasone + pomalidomide (SPd); enrollment complete * Arm 2: Selinexor + dexamethasone + bortezomib (SVd); enrollment complete * Arm 3: Selinexor + dexamethasone + lenalidomide (SRd) in RRMM; enrollment complete * Arm 4: Selinexor + dexamethasone + pomalidomide + bortezomib (SPVd); enrollment complete * Arm 5: Selinexor + dexamethasone + daratumumab (SDd); enrollment complete * Arm 6: Selinexor + dexamethasone + carfilzomib (SKd); enrollment complete * Arm 7: Selinexor + dexamethasone + lenalidomide (SRd) in NDMM; enrollment complete * Arm 8: Selinexor + dexamethasone + ixazomib (SNd); enrollment complete * Arm 9: Selinexor + dexamethasone + pomalidomide + elotuzumab (SPEd); enrollment complete * Arm 10: Selinexor + dexamethasone + belantamab mafodotin (SBd); enrollment complete * Arm 11: Selinexor + dexamethasone + pomalidomide + daratumumab (SDPd); enrollment complete * Arm 12: Selinexor + dexamethasone + mezigdomide (SMd); actively recruiting Selinexor pharmacokinetics: * PK Run-in (Days 1-14): Starting in protocol version 8.0, patients enrolled to any arm in the Dose Escalation Phase (i.e., Arm 4 \[SPVd\], Arm 6 \[SKd\], Arm 8 \[SNd\], Arm 9 \[SPEd\], Arm 10 \[SBd\], and Arm 11 \[SDPd\]) will also first be enrolled to a pharmacokinetics (PK) Run-in period until 9 patients have been enrolled to this period to evaluate the PK of selinexor before and after co-administration with a strong CYP3A4 inhibitor. This run-in period does not apply to Arm 12 (SMd).
Locations & Contact
Fill out the form and "Notify Multiple Myeloma Research Foundation" to let the Multiple Myeloma Research Foundation know you are interested in this trial.
Contacts: