MagnetisMM-5: Study of Elranatamab (PF-06863135) Monotherapy and Elranatamab + Daratumumab Versus Daratumumab + Pomalidomide + Dexamethasone in Participants With Relapsed/Refractory Multiple Myeloma
Summary
Trial Summary
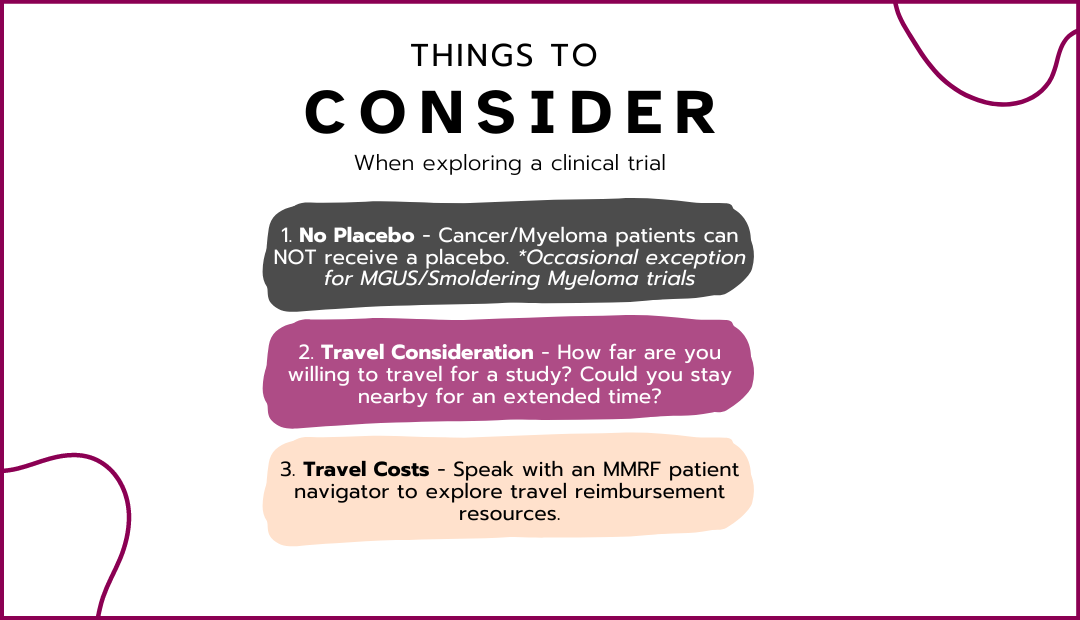
The purpose of this study is to evaluate whether the BCMA-CD3 bispecific antibody elranatamab, alone and/or in combination with the anti-CD38 monoclonal antibody, daratumumab, can provide more benefit to people with multiple myeloma compared to a combination therapy including daratumumab, pomalidomide, and dexamethasone. People with multiple myeloma who have received previous treatment including lenalidomide and a proteasome inhibitor will be enrolled in the study. Part 1 of the study will assess the safety and activity of different doses of elranatamab in combination with daratumumab. People participating in Part 2 of the study will be randomly assigned to receive either elranatamab alone, elranatamab plus daratumumab, or daratumumab, pomalidomide, and dexamethasone. Part 2 will compare the safety and activity of (1) elranatamab alone compared to daratumumab, pomalidomide, and dexamethasone, and (2) elranatamab plus daratumumab compared to daratumumab, pomalidomide, and dexamethasone. Participants in all parts of the study will receive study treatment until their disease progresses, they experience unacceptable side effects, or they choose to no longer participate in the study.
The purpose of this study is to evaluate whether the BCMA-CD3 bispecific antibody elranatamab, alone and/or in combination with the anti-CD38 monoclonal antibody, daratumumab, can provide more benefit to people with multiple myeloma compared to a combination therapy including daratumumab, pomalidomide, and dexamethasone. People with multiple myeloma who have received previous treatment including lenalidomide and a proteasome inhibitor will be enrolled in the study. Part 1 of the study will assess the safety and activity of different doses of elranatamab in combination with daratumumab. People participating in Part 2 of the study will be randomly assigned to receive either elranatamab alone, elranatamab plus daratumumab, or daratumumab, pomalidomide, and dexamethasone. Part 2 will compare the safety and activity of (1) elranatamab alone compared to daratumumab, pomalidomide, and dexamethasone, and (2) elranatamab plus daratumumab compared to daratumumab, pomalidomide, and dexamethasone. Participants in all parts of the study will receive study treatment until their disease progresses, they experience unacceptable side effects, or they choose to no longer participate in the study.
Locations & Contact
Fill out the form and "Notify Multiple Myeloma Research Foundation" to let the Multiple Myeloma Research Foundation know you are interested in this trial.
Contacts:
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Name: Pfizer CT.gov Call Center
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021
Email: ClinicalTrials.gov_Inquiries@pfizer.com
Phone: 1-800-718-1021